Let’s work together
Whether you want to explore the Onesum Platform or engage our expert consultants, we’d love to learn about your thoughts and challenges.

Critically, the platform addresses key limitations of LLMs in scientific reporting by embedding expert oversight at every step. Each report component is designed to support rigorous review, ensuring scientific defensibility and regulatory alignment.
⏱️ Reports that once required days or even months to complete can now be finalized within hours. Highly customizable, the platform can be configured to generate virtually any report type, including those intended for regulatory submission.
We welcome collaboration; if there’s a specific report you’d like to develop together, let us know!
Standardized reports and databases are continuously generated across R&D, guiding research decisions and enabling regulatory submissions. For example, in toxicological risk assessment, several standardized reports are routinely developed to support decision-making and regulatory alignment, including:
The Figure below illustrates the stepwise workflow within the Onesum platform for generating standardized, regulatory-aligned reports. The process begins by specifying the report type and entering foundational information about its scope and focus. This is followed by a comprehensive search across web sources and other databases to identify relevant documents and supporting data. In parallel, LLMs may be used to suggest additional materials for consideration. This document collection, integrated with Oneum’s capabilities in content structuring and analysis, enables the generation of expert-reviewed report sections and the seamless export of full reports to Word.

Permitted Daily Exposures (PDEs) are a subset of Health-Based Guidance Values (HBGVs) that define safe thresholds for daily chemical exposure. Within the context of Extractables and Leachables (E&Ls), PDEs are established by integrating data from proprietary toxicology studies, peer-reviewed literature, and authoritative regulatory sources such as ECHA, EFSA, and EPA.
To initiate a PDE report, users begin by selecting the appropriate report type and defining key parameters, such as the test chemical, illustrated here:
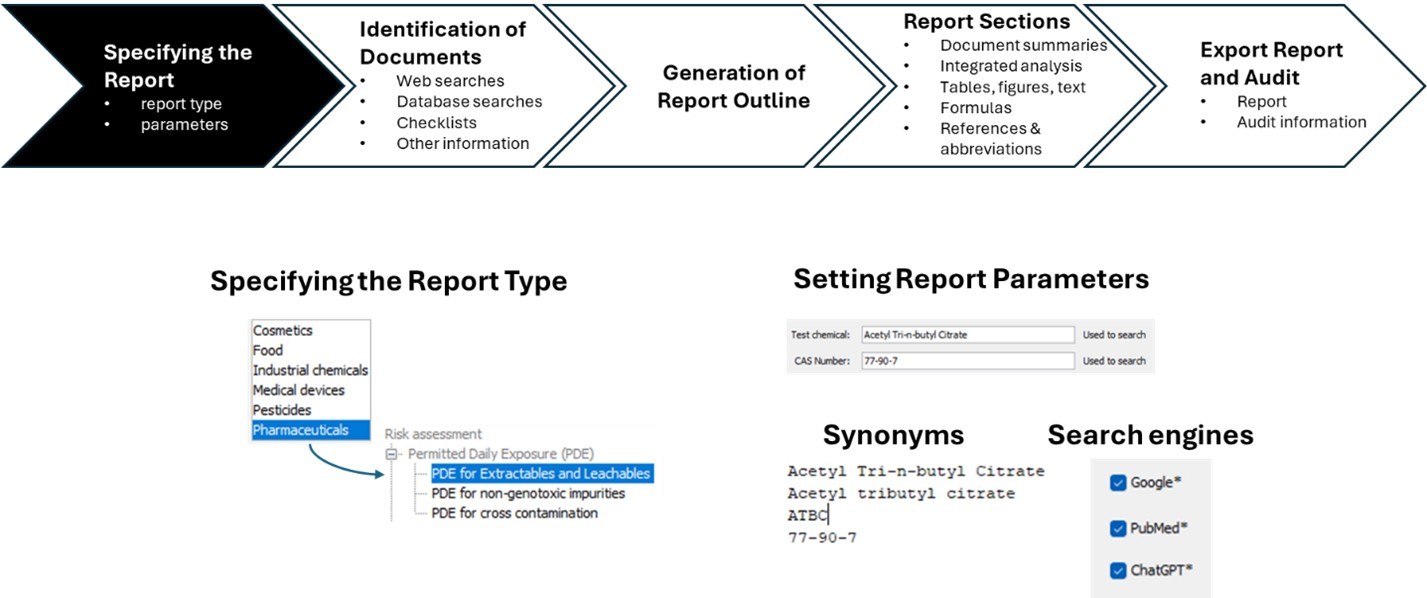
A combination of targeted web searches and database queries is then executed using predefined, domain-specific prompts to identify documents and content relevant to report generation. Once reviewed, selected documents can be uploaded and incorporated into the report through summarization tools. Additionally, curated checklists of authoritative websites can be browsed to locate supplementary information. Relevant documents from these sources may be uploaded to enrich the report’s knowledge base along with proprietary information.
The figure below illustrates the range of tools and options available for generating and finalizing report sections.

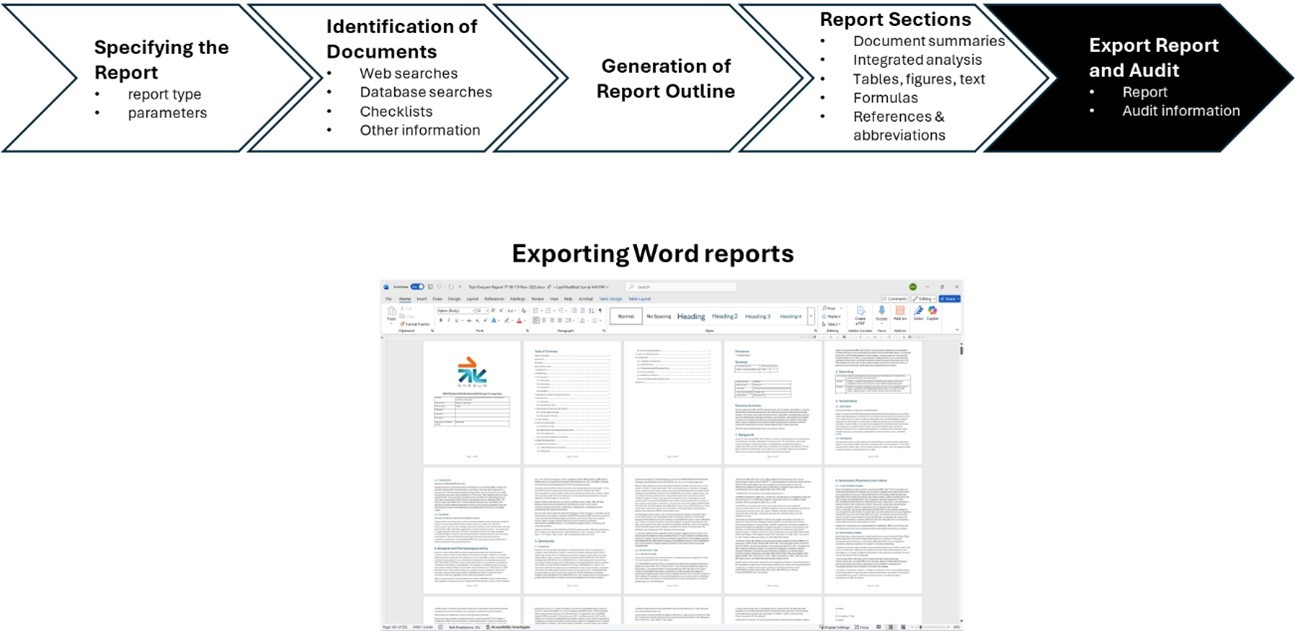
Finally, upon completion, the full report and optionally its associated audit trail can be exported as a Word document, as illustrated below:
Whether you want to explore the Onesum Platform or engage our expert consultants, we’d love to learn about your thoughts and challenges.

contact@onesumportal.com